BioAge
Founded Year
2015Stage
IPO | IPOTotal Raised
$297MDate of IPO
9/26/2024Market Cap
0.28BStock Price
8.01Revenue
$0000About BioAge
BioAge offers clinical-stage biotechnology focused on developing therapies for metabolic diseases by targeting human aging. It has programs aimed at treating conditions like obesity and related metabolic disorders. It serves the healthcare and pharmaceutical sectors with its drug discovery and development. It was founded in 2015 and is based in Richmond, California.
Loading...
BioAge's Products & Differentiators
BGE-117
(Please note that this and all drugs developed by BioAge are pre-market and have not yet been approved by FDA). BGE-117 is a HIF-PH inhibitor that activates hypoxia signaling, increasing the body’s ability to synthesize hemoglobin, the molecule that carries oxygen in the blood. Because BGE-117 targets a critical pathway that is dysregulated as we age, it holds great promise for anemia of aging as well as several acute and chronic conditions driven by muscle aging.
Loading...
Research containing BioAge
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned BioAge in 4 CB Insights research briefs, most recently on Oct 22, 2025.
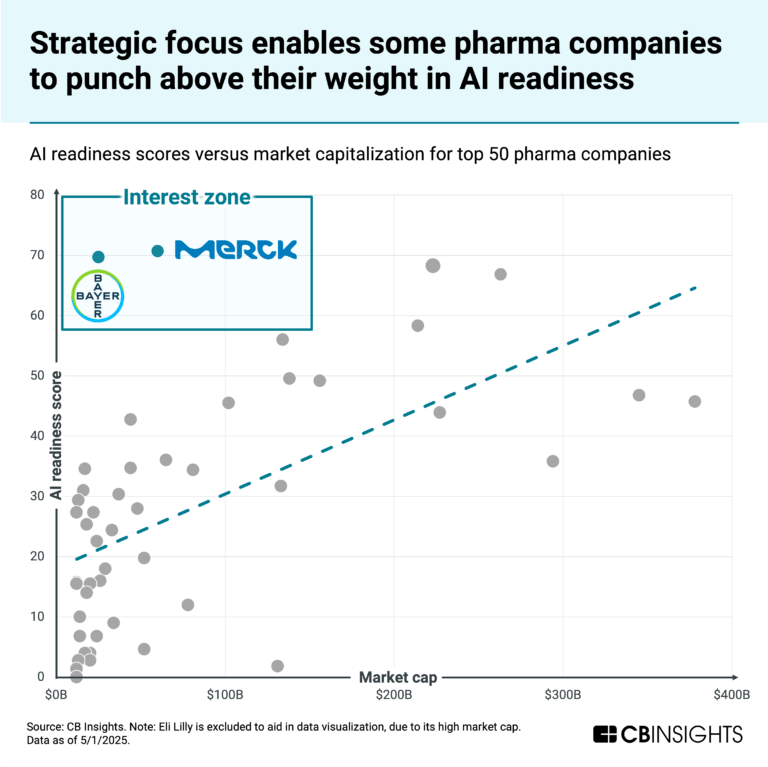
Oct 22, 2025
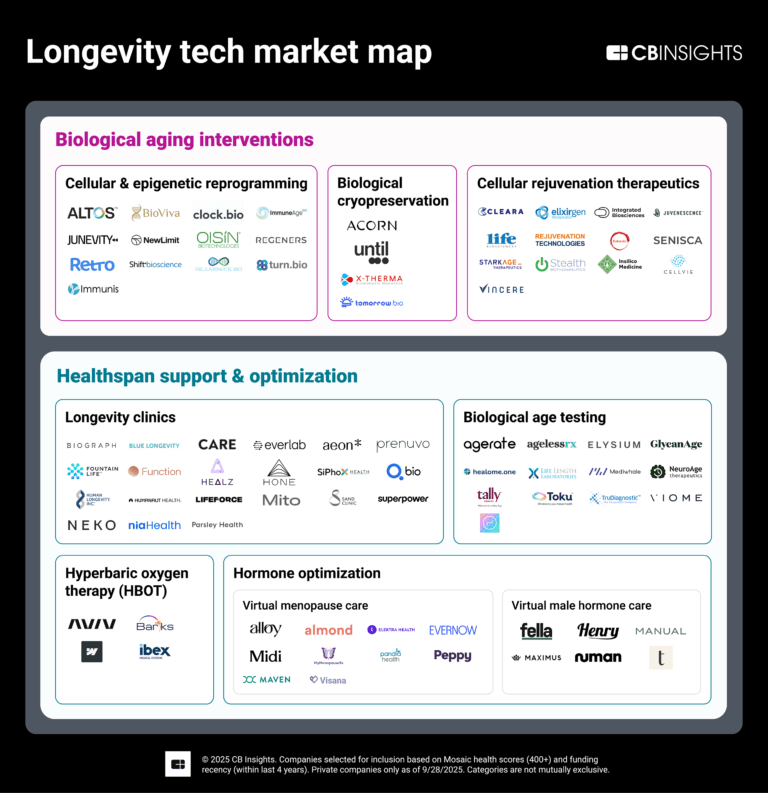
The longevity tech market map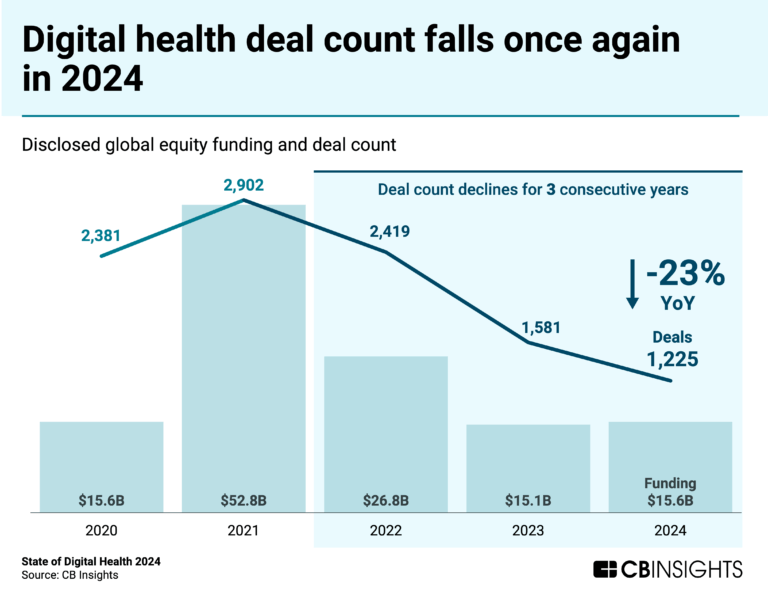
Jan 16, 2025 report
State of Digital Health 2024 Report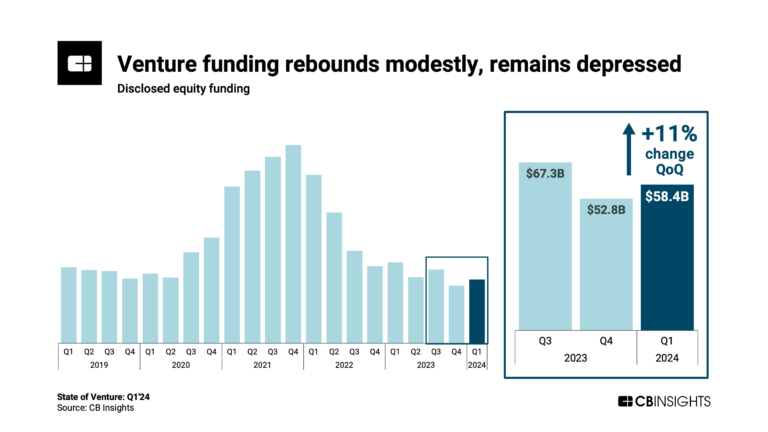
Apr 4, 2024 report
State of Venture Q1’24 ReportExpert Collections containing BioAge
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
BioAge is included in 2 Expert Collections, including Digital Health.
Digital Health
12,122 items
The digital health collection includes vendors developing software, platforms, sensor & robotic hardware, health data infrastructure, and tech-enabled services in healthcare. The list excludes pureplay pharma/biopharma, sequencing instruments, gene editing, and assistive tech.
Artificial Intelligence (AI)
20,894 items
BioAge Patents
BioAge has filed 23 patents.
The 3 most popular patent topics include:
- rare diseases
- immune system
- immunology

Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
7/17/2023 | 2/4/2025 | Inflammations, Rare diseases, Immune system, Immunology, Clusters of differentiation | Grant |
Application Date | 7/17/2023 |
|---|---|
Grant Date | 2/4/2025 |
Title | |
Related Topics | Inflammations, Rare diseases, Immune system, Immunology, Clusters of differentiation |
Status | Grant |
Latest BioAge News
Nov 11, 2025
To embed, copy and paste the code into your website or blog: <iframe frameborder="1" height="620" scrolling="auto" src="//www.jdsupra.com/post/contentViewerEmbed.aspx?fid=35460487-7493-4f3a-a8f1-5e69d2c01259" style="border: 2px solid #ccc; overflow-x:hidden !important; overflow:hidden;" width="100%"></iframe> On October 30, 2025, Chief Judge Richard Seeborg of the United States District Court of the Northern District of California granted a motion to dismiss a putative securities class action alleging a biopharmaceutical company (the “Company”) and its officers (“Individual Defendants” and, collectively, “Defendants”) violated Sections 11 and 15 of the Securities Act of 1933 (the “Securities Act”). Soto v. BioAge Labs, Inc., et al., No. 25-cv-00196-RS (N.D. Cal. Oct. 30, 2025). Plaintiffs alleged that Defendants misled investors by omitting from risk disclosures an allegedly “inevitable” side-effect of the Company’s leading drug candidate. The Court dismissed the claims, finding (1) Defendants did not have any obligation under Section 11 to make the allegedly absent disclosure and (2) plaintiffs failed to plausibly allege the side-effect was inevitable. The Company holds an exclusive license to research, develop, and commercialize a weight-loss drug that is its leading product. Roughly two months before the Company’s initial public offering (“IPO”), it began a Phase 2 clinical trial on that drug. At the time of the IPO, the Company warned in its offering documents that “unexpected” or “atypical adverse events or side-effects” discovered during the ongoing trial could pose a risk to the Company and the drug’s developmental efforts. Approximately nine weeks after the IPO, the Company announced that some Phase 2 trial patients allegedly developed transaminitis (or elevated liver enzyme levels), prompting the Company to discontinue the trial and abandon the drug’s development. Plaintiffs alleged that transaminitis was a “typical” and “virtually certain” side-effect given the trial’s design and the drug’s properties and that Defendants’ failure to disclose this information caused their investments in the Company to suffer. The Court first held that, because the Company did not speak in its offering documents about transaminitis—or otherwise minimize a specific, known risk of the condition—Plaintiffs could not identify any statement rendered misleading by that alleged omission as required to state a Section 11 claim. The Court also rejected plaintiffs’ implication theory—that by disclosing possible “atypical” side-effects, the Company suggested no “typical” side effects posed a risk—as incompatible with In re Rigel Pharms., Inc., 687 F.3d 869 (9th Cir. 2012), which forecloses the notion an issuer must “disclose everything on a certain topic once [the issuer] disclose[s] anything on that topic” to avoid Section 11 liability. Separately, the Court held plaintiffs did not plead facts supporting a reasonable inference that transaminitis was “inevitable.” The Court found that the data from a pre-clinical animal study and the Phase 1 trial did not show transaminitis was likely, let alone “inevitable.” As to plaintiffs’ Phase 2 design-flaw allegations—such as that the Company enrolled participants who might have had elevated liver enzymes due to environmental and life-style factors—the Court found it could infer at most a possibility, not a near certainty, transaminitis would emerge to derail the trial. Concluding that the Section 11 claim failed, the Court did not address the Section 15 claim, but granted Plaintiffs leave to amend their complaint. Links & Downloads
BioAge Frequently Asked Questions (FAQ)
When was BioAge founded?
BioAge was founded in 2015.
Where is BioAge's headquarters?
BioAge's headquarters is located at 1445A South 50th Street, Richmond.
What is BioAge's latest funding round?
BioAge's latest funding round is IPO.
How much did BioAge raise?
BioAge raised a total of $297M.
Who are the investors of BioAge?
Investors of BioAge include Felicis, Andreessen Horowitz, Amgen Ventures, RA Capital Management, Lilly Ventures and 23 more.
Who are BioAge's competitors?
Competitors of BioAge include Brightseed and 4 more.
What products does BioAge offer?
BioAge's products include BGE-117 and 2 more.
Loading...
Compare BioAge to Competitors

Atomwise develops machine learning-based discovery engines and uses artificial intelligence (AI)-based neural networks to help discover new medicines. It predicts drug candidates for pharmaceutical companies, start-ups, and research institutions, and designs drugs using computational drug design. It was formerly known as Chematria. The company was founded in 2012 and is based in San Francisco, California.

Canomiks is a technology company focused on the functional food, beverage, dietary supplement, and cosmeceutical industries. The company offers a genomics and AI-based platform that develops new formulations and assesses the biological efficacy and safety of ingredients and formulations. Canomiks serves the functional food and beverage, dietary supplement, and skincare industries. It was founded in 2016 and is based in Rochester, Minnesota.

Rockfish Bio focuses on the development of senolytic therapies within the biotechnology sector. The company's main offerings include compounds designed to treat age-related diseases by targeting and eliminating senescent cells to promote healthy aging. Rockfish Bio's therapies are applicable to a range of conditions such as chronic kidney disease, liver fibrosis, and neurodegenerative diseases. It was founded in 2021 and is based in Wien, Austria.

Viome operates as a health and longevity company. It specializes in preventing, diagnosing, and reversing chronic diseases and aging through its at-home health tests and personalized nutrition. The company's main offerings include at-home microbiome health tests that utilize advanced RNA sequencing technology to generate health scores and nutrition recommendations tailored to an individual's unique biology. It was founded in 2016 and is based in Bellevue, Washington.
Indena is a company dedicated to the identification, development and production of active principles derived from plants, for use in the pharmaceutical, health-food and cosmetics industries. Indena, a privately owned Italian company, has around 700 employees, including 10% dedicated to full-time research, aims to manage cultivation, manufacturing, and distribution operations in more than 40 countries throughout the world. The key to Indena's success is its research, covering: the screening of medicinal plants for their pharmacological benefits; the identification of new active principles; and the development of extraction and purification systems at the cutting-edge of industrial application. The phyto-chemical research is carried out in Indena's own Research Center in Settala, Italy. Indena also co-operates with the world's most prestigious universities and private research institutions in the biological assessment of safety and effectiveness up to clinical phase I. One of the main strategic objectives of the research is the development of new active principles for pharmaceutical applications. This is also an indispensable base for the health-food and cosmetics industries. In the pharmaceutical sector Indena is currently focusing on the development of anti-cancer treatments, following the successes of paclitaxel, as well as on anti-microbial and on anti-viral drugs. Also under study are active principles for the treatment of pathologies and dysfunction of the central nervous system, such as depression, anxiety, impotence, sleep disorders, and algesia. Today Indena is also concentrating its efforts on the industrial production of high-quality standardised active principles derived from plants, which are important ingredients in health food products. In fact, the company produces standardised extracts from edible plants that are traditionally recognised as having therapeutic properties, as well as from plants that have proven pharmacological value. Indena's research and production activities are particularly attentive to plant-based principles that have anti-oxidant properties and are effective in preventing damage caused by free radicals. Backed by over 80 years of botanical experience, the Italian company has developed a plantation network, managed by experts, to supply its research and production centers with officinal plants, while at the same time ensuring bio-diversity and protecting the ecosystem balance from uncontrolled harvesting. Today more than 60% of the raw material used in the manufacturing process comes from cultivation. Indena's decades-long global presence in the plant extraction industry is a guarantee of its in-depth knowledge and understanding of the business, market trends and legal considerations, through constant communication and interaction between the company's experts and the major international regulatory authorities such as WHO, EMEA, and ESCOP, and main pharmacopoeias.

Onegevity is a consumer health intelligence company, which combines a multi-omic AI platform with consumer-friendly products and digital services. The Health Intelligence platform can accept inputs from lab work, wearables, test results, clinical intake data, and virtually any other health and wellness information. From there, it uses AI to “map” the complex and dynamic metabolic outputs from an individual and compare to individuals alike. It can provide personalized lifestyle recommendations including nutrition, supplements, exercise/lifestyle, and can further recommend individualized follow-up testing, educational needs, and more. The platform can be used by an individual, a health-care practitioner, or for corporate wellness purposes.
Loading...