
Freenome
Founded Year
2014Stage
Series F | AliveTotal Raised
$1.353BLast Raised
$254M | 2 yrs agoMosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
-7 points in the past 30 days
About Freenome
Freenome operates as a biotechnology company specializing in early cancer detection within the healthcare sector. The company develops blood tests that use multiomics technology and artificial intelligence to identify cancer at its earliest and most treatable stages. It was founded in 2014 and is based in Brisbane, California.
Loading...
ESPs containing Freenome
The ESP matrix leverages data and analyst insight to identify and rank leading companies in a given technology landscape.
The liquid biopsy cancer screening market offers a non-invasive alternative to traditional tissue biopsies for detecting cancer. This market includes various solutions such as circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) tests, which can detect cancer at an early stage and monitor treatment effectiveness. The liquid biopsy method is less invasive, faster, and more cost-effectiv…
Freenome named as Challenger among 15 other companies, including Roche, Becton Dickinson, and Exact Sciences.
Loading...
Research containing Freenome
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Freenome in 7 CB Insights research briefs, most recently on Jan 16, 2025.
Jan 16, 2025 report
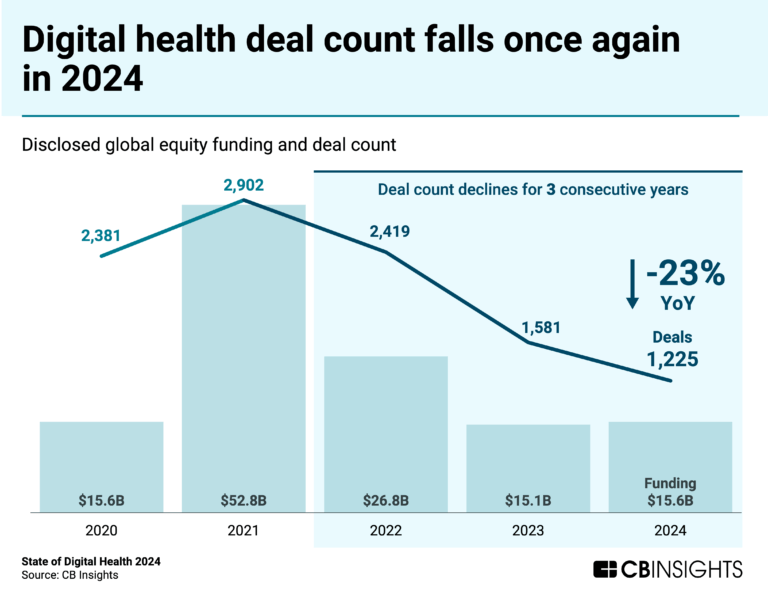
State of Digital Health 2024 Report
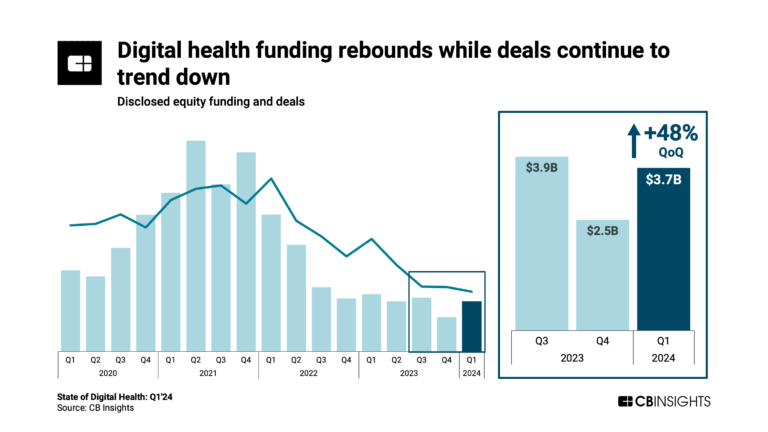
Apr 25, 2024 report
State of Digital Health Q1’24 Report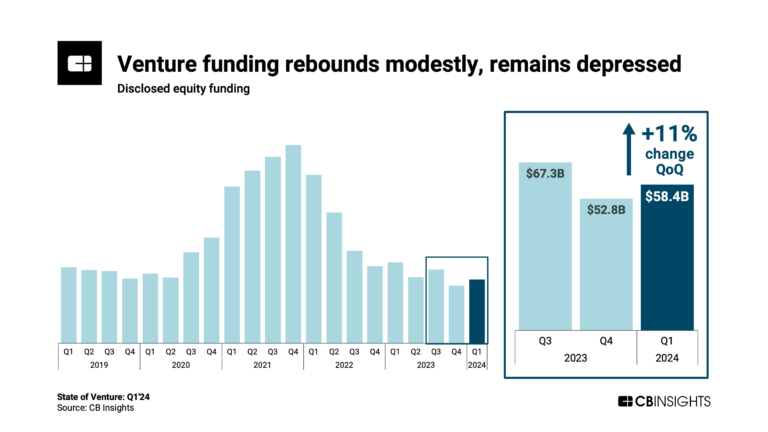
Apr 4, 2024 report
State of Venture Q1’24 Report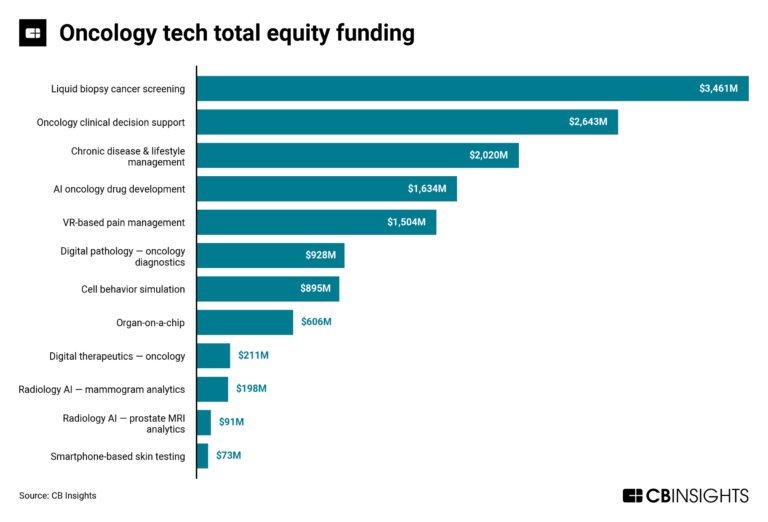
Aug 10, 2023
The oncology tech market map
Aug 1, 2023
The state of healthcare AI in 5 chartsExpert Collections containing Freenome
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Freenome is included in 8 Expert Collections, including Unicorns- Billion Dollar Startups.
Unicorns- Billion Dollar Startups
1,309 items
AI 100 (All Winners 2018-2025)
99 items
Winners of CB Insights' annual AI 100, a list of the 100 most promising AI startups in the world.
Conference Exhibitors
5,501 items
HLTH is a healthcare event bringing together startups and large companies from pharma, health insurance, business intelligence, and more to discuss the shifting landscape of healthcare
Digital Health 50
300 items
The most promising digital health startups transforming the healthcare industry
Digital Health
12,122 items
The digital health collection includes vendors developing software, platforms, sensor & robotic hardware, health data infrastructure, and tech-enabled services in healthcare. The list excludes pureplay pharma/biopharma, sequencing instruments, gene editing, and assistive tech.
Precision Medicine Tech Market Map
160 items
This CB Insights Tech Market Map highlights 160 precision medicine companies that are addressing 9 distinct technology priorities that pharmaceutical companies and healthcare providers face.
Freenome Patents
Freenome has filed 22 patents.
The 3 most popular patent topics include:
- molecular biology
- oncology
- cancer screening
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
8/18/2023 | 3/11/2025 | Clusters of differentiation, Blood tests, Blood, Immunology, Cell biology | Grant |
Application Date | 8/18/2023 |
|---|---|
Grant Date | 3/11/2025 |
Title | |
Related Topics | Clusters of differentiation, Blood tests, Blood, Immunology, Cell biology |
Status | Grant |
Latest Freenome News
Nov 11, 2025
Freenome Appoints Industry Veteran Rob Guigley as Chief Commercial Officer November 11, 2025 Freenome building commercial infrastructure following recent CLIA lab certification and early access program for key health system partners, with new test launches for colorectal cancer, lung cancer and other indications planned in 2026 In previously announced deal, Exact Sciences acquired exclusive U.S. licensing and commercialization rights to Freenome’s blood-based colorectal cancer screening tests for up to $885M Freenome, an early cancer detection company developing blood-based tests to identify cancer at its most treatable stage, today announced the appointment of industry veteran Rob Guigley as its chief commercial officer. This key leadership addition will spearhead the execution of the exclusive U.S. licensing and commercialization that leverages Exact Sciences’ extensive commercial footprint to accelerate access to Freenome’s colorectal cancer (CRC) blood-based screening test. In addition, Guigley will lead the build-out of Freenome’s core commercial capabilities and activities for our broader suite of future tests and platform offerings. The waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976 (HSR) has expired, which satisfies one of the conditions for closing the transaction that was initially announced on Aug. 6, 2025. The U.S. Food and Drug Administration (FDA) is currently reviewing the Premarket Approval Application for the initial version of the test, known as SimpleScreen™ CRC in the FDA filing, with approval expected in the second half of 2026. “As we transition to this new phase, we are focused on strengthening our commercial leadership to maximize the reach of our CRC test while aggressively advancing our multi-cancer pipeline and generation of real-world data (RWD) through commercial testing,” said Aaron Elliott, Ph.D., chief executive officer of Freenome. “Rob Guigley has consistently demonstrated the ability to design and scale commercial organizations in the primary care field, and his experience will be invaluable in preparing Freenome for future clinical impact.” Guigley joins the company with more than 20 years of experience leading commercial organizations across diagnostics, digital health and healthcare technology, including at Delfi Diagnostics, Invitae, Ambry Genetics and Omada Health. He will lead Freenome’s go-to-market strategy with health systems, including developing wraparound services to empower caregivers and integrating the company’s tests into primary care workflows. Guigley’s experience with digital products enabled by RWD strategically aligns with the Exact Sciences commercial relationship, which provides Freenome with access to all multimodal data from patients to power future AI/ML models across multiple cancer indications. Freenome intends to launch a laboratory-developed test (LDT) version of its lung cancer screening test in the second half of 2026. More than 10 other cancer indications embedded in the current assay are also being pursued, informed by data from the fully enrolled, multi-cancer Vallania and Sanderson studies. “What drew me to Freenome is the combination of rigorous science, clinical validation and platform strength protected by a growing data moat, paired with a clear vision for making early detection accessible to everyone,” Guigley said. “I look forward to working with Exact Sciences to accelerate access to SimpleScreen CRC following FDA approval, and I’m excited to build Freenome’s commercial infrastructure to bring our innovative personalized multi-cancer test portfolio to the market.” Freenome’s multiomics discovery platform evaluates multiple biomarker classes, including epigenomics, transcriptomics, metabolomics and proteomics, to identify the early biological signals of disease in the bloodstream. The company’s goal is to create a common lab platform with custom panels and classifiers to offer multiple tests to an individual based on health profiles and guideline eligibility. Targeting high-, elevated-, and average-risk populations based on the unmet need allows the tests to be optimized for higher sensitivity at clinically acceptable specificity. Select health systems are currently being evaluated for a SimpleScreen CRC early access program that is launching in January 2026, and Freenome expects the first patient samples to arrive at its clinical laboratory that month. The company’s lab received its Clinical Laboratory Improvement Amendments (CLIA) certification in October, indicating it meets federal quality standards for accuracy and reliability. PR Newswire PR Newswire empowers communicators to identify and engage with key influencers, craft and distribute meaningful stories, and measure the financial impact of their efforts. Cision is a leading global provider of earned media software and services to public relations and marketing communications professionals. previous post
Freenome Frequently Asked Questions (FAQ)
When was Freenome founded?
Freenome was founded in 2014.
Where is Freenome's headquarters?
Freenome's headquarters is located at 3300 Marina Boulevard, Brisbane.
What is Freenome's latest funding round?
Freenome's latest funding round is Series F.
How much did Freenome raise?
Freenome raised a total of $1.353B.
Who are the investors of Freenome?
Investors of Freenome include DCVC, Andreessen Horowitz, T. Rowe Price, Section 32, Polaris Partners and 55 more.
Who are Freenome's competitors?
Competitors of Freenome include Owlstone Medical, 20/20 BioLabs, BioTwin, GRAIL, Diagu and 7 more.
Loading...
Compare Freenome to Competitors

20/20 BioLabs provides diagnostics for the healthcare sector, focusing on the detection and prevention of cancers and chronic diseases. The company offers laboratory-based blood tests that utilize machine learning algorithms to analyze real-world data, providing testing solutions. It serves individuals seeking health management and healthcare providers in need of diagnostic tools. 20/20 BioLabs was formerly known as 20/20 GeneSystems. It was founded in 2000 and is based in Gaithersburg, Maryland.

Owlstone Medical focuses on breath analysis for disease detection within the healthcare sector. The company develops technology for discovering and validating biomarkers in breath, employing chemical analysis and sensor technology for diagnosis. Owlstone Medical's products and services are intended for academic, clinical, and pharmaceutical research partners involved in developing breath-based diagnostics. It was founded in 2004 and is based in Cambridge, United Kingdom.
OncoTAb specializes in biotechnology with a focus on cancer diagnostic solutions. The company offers Agkura® Personal Score, a non-invasive blood test that measures tumor protein levels to aid in breast cancer detection. This test serves as a supplemental tool to mammography, which often misses cancers in women with dense breast tissue. It is based in Charlotte, North Carolina.

AccuraGen develops liquid biopsy technology for the cancer diagnostics industry. The company provides services related to DNA and RNA sequencing to assist in treatment plans for cancer patients, emphasizing non-invasive diagnostic methods. AccuraGen serves the healthcare sector, particularly in oncology. It is based in Menlo Park, California.
Liquid Genomics provides testing services for the detection of cancer gene mutations through blood-based testing within the healthcare and biotechnology sectors. The company utilizes digital PCR and allele-specific blocker PCR technologies to identify and quantify mutations in blood or tissue samples. Its services are aimed at the healthcare industry, focusing on cancer diagnostics and research. It is based in Torrance, California.
Artificial Intelligence Expert focuses on AI technologies related to early detection of cancer within the healthcare sector. The company provides tests that can identify various types of cancer with reported accuracy rates. It is based in Cluj-Napoca, Romania.
Loading...
