Insilico Medicine
Founded Year
2014Stage
Series E | AliveTotal Raised
$524.8MValuation
$0000Last Raised
$123M | 10 mos agoRevenue
$0000Mosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
+40 points in the past 30 days
About Insilico Medicine
Insilico Medicine focuses on artificial intelligence in drug discovery and development. The company provides artificial intelligence platforms for multi-omics target discovery, deep biology analysis, automated drug design, and predicting outcomes in clinical trials. The technologies are intended to assist in the process of discovering and developing new medications for various diseases. It was founded in 2014 and is based in New Territories, Hong Kong.
Loading...
Insilico Medicine's Product Videos

ESPs containing Insilico Medicine
The ESP matrix leverages data and analyst insight to identify and rank leading companies in a given technology landscape.
The cellular rejuvenation therapeutics market includes companies developing therapies that target key hallmarks of aging such as cellular senescence, telomere attrition, and mitochondrial dysfunction. These interventions include senolytics that remove damaged cells, telomere-targeting therapies that maintain chromosome integrity, and mitochondrial treatments that optimize cellular energy productio…
Insilico Medicine named as Leader among 15 other companies, including Juvenescence, Rubedo, and Shift Bioscience.
Insilico Medicine's Products & Differentiators
Pharma.AI
Global’s first end-to-end generative Al software and robotics platform designed to improve the quality and productivity of pharmaceutical research, currently commercially-available
Loading...
Research containing Insilico Medicine
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Insilico Medicine in 21 CB Insights research briefs, most recently on Sep 19, 2025.

Sep 19, 2025 report
Book of Scouting Reports: AI Drug Discovery
Aug 22, 2025 report
Book of Scouting Reports: Generative AI in Healthcare & Life Sciences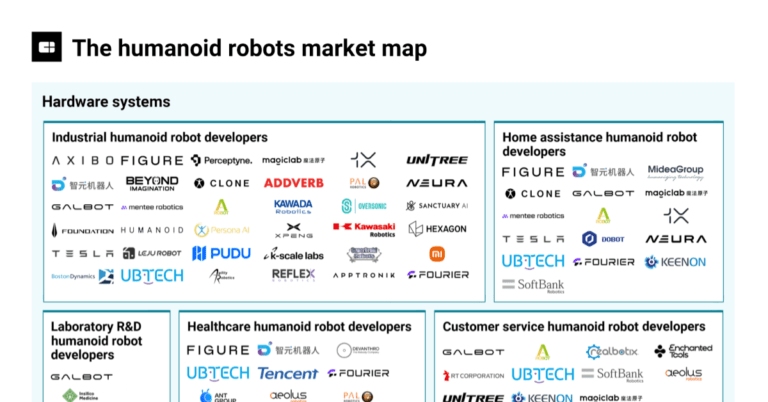
Jun 26, 2025
The humanoid robots market map
Jun 26, 2025 report
Book of Scouting Reports: Humanoid Robots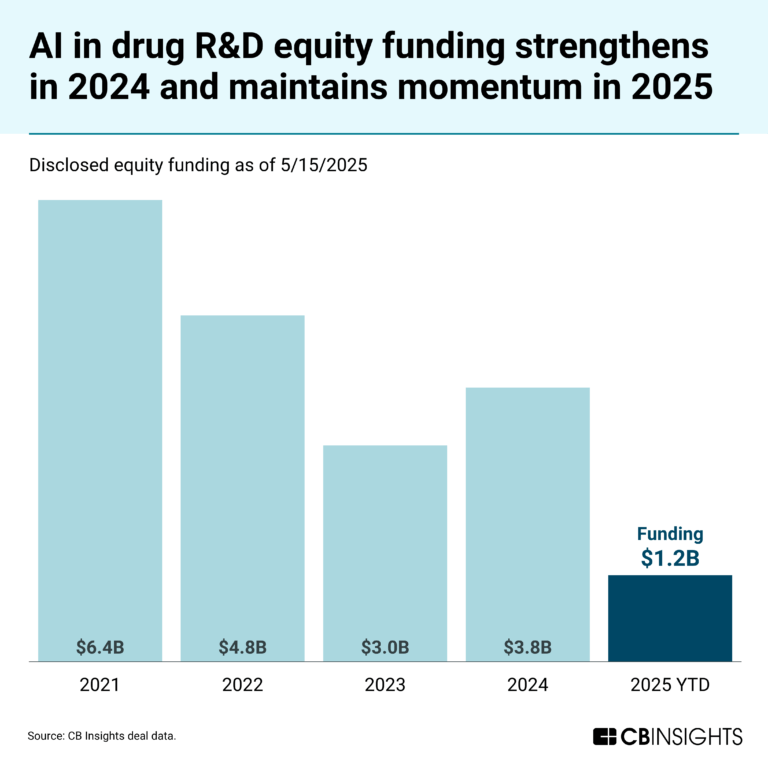
May 23, 2025
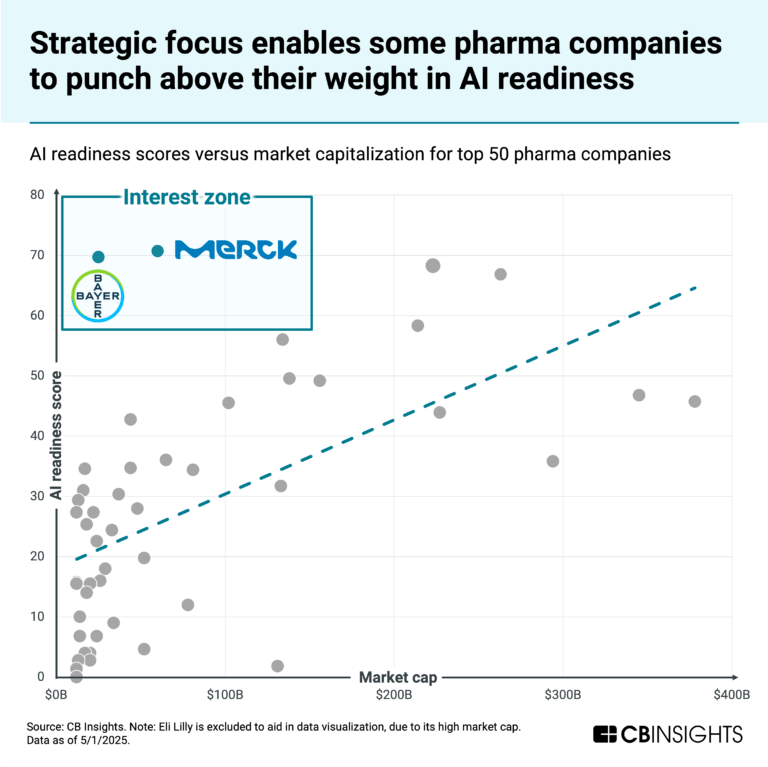
The AI in drug R&D market map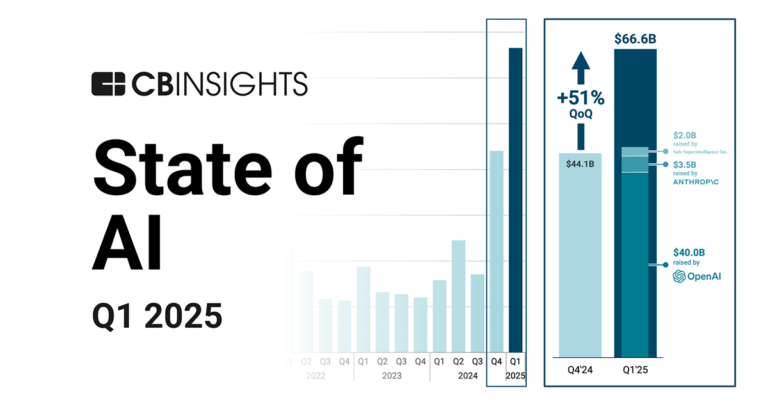
May 1, 2025 report
State of AI Q1’25 Report
Apr 17, 2025 report
State of Digital Health Q1’25 ReportExpert Collections containing Insilico Medicine
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Insilico Medicine is included in 7 Expert Collections, including AI 100 (All Winners 2018-2025).
AI 100 (All Winners 2018-2025)
199 items
Winners of CB Insights' annual AI 100, a list of the 100 most promising AI startups in the world.
Digital Health 50
150 items
The winners of the second annual CB Insights Digital Health 150.
Digital Health
12,122 items
The digital health collection includes vendors developing software, platforms, sensor & robotic hardware, health data infrastructure, and tech-enabled services in healthcare. The list excludes pureplay pharma/biopharma, sequencing instruments, gene editing, and assistive tech.
Drug Discovery Tech Market Map
221 items
This CB Insights Tech Market Map highlights 220 drug discovery companies that are addressing 12 distinct technology priorities that pharmaceutical companies face.
Generative AI
2,951 items
Companies working on generative AI applications and infrastructure.
AI in drug discovery
528 items
Companies using AI to advance therapeutic discovery, categorized into: platforms (primary product is software) and discovery engines (primary product is therapeutics). Additional funnel descriptions reflect how companies are applying AI.
Insilico Medicine Patents
Insilico Medicine has filed 71 patents.
The 3 most popular patent topics include:
- transcription factors
- proteins
- clusters of differentiation

Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
7/6/2023 | 2/18/2025 | Trifluoromethyl compounds, Transcription factors, Designer drugs, Prodrugs, Cannabinoids | Grant |
Application Date | 7/6/2023 |
|---|---|
Grant Date | 2/18/2025 |
Title | |
Related Topics | Trifluoromethyl compounds, Transcription factors, Designer drugs, Prodrugs, Cannabinoids |
Status | Grant |
Latest Insilico Medicine News
Nov 18, 2025
U.S. Drives Surge in In-Silico Clinical Trials | Global Market Set to Hit $6.39B by 2033 with Rapid Digital R&D Adoption” — DataM Intelligence 4Market Research LLP AUSTIN, TX, UNITED STATES, November 18, 2025 / EINPresswire.com / -- Market Overview According to DataM Intelligence research report 2025. in-silico clinical trials market was valued at USD 3.76 billion in 2023 and increased to USD 3.95 billion in 2024. It is projected to reach USD 6.39 billion by 2033, growing at a CAGR of 5.5% during the forecast period 2025–2033.. In-silico trials utilize computer simulations and modeling to predict drug efficacy and safety, reducing costs and accelerating drug development. Download PDF Brochure: https://www.datamintelligence.com/download-sample/in-silico-clinical-trials-market Recent Clinical Trials 1. In-silico cohorts and virtual patients increasingly supplement small Phase I or proof-of-concept studies, improving trial design, patient stratification, and early dose selection. 2. AstraZeneca utilized Quantitative Systems Pharmacology (QSP) models and virtual patients to accelerate PCSK9 therapy development, enabling a 6-month head start to Phase 2 trials. 3. Insilico Medicine initiated global multicenter Phase 1 dosing for its AI-designed cancer drug ISM3412, progressing rapidly leveraging in-silico validation of safety and pharmacokinetics. 4. Computational fluid dynamics (CFD) virtual trials predicted aneurysm flow reduction for Medtronic's Pipeline Embolization Device, correlating well with real clinical trial outcomes. Key Highlights from This Report . North America led the in-silico clinical trials market in 2024, accounting for the highest revenue share at 43.5%. . The Asia Pacific region is the fastest-growing market, projected to record the highest CAGR of 7.4% during the forecast period. . Pharmacokinetic and pharmacodynamic (PK/PD) models dominated the market by model type, representing a 39.3% share in 2024. . The drug development segment held the largest share by application, capturing 41.3% of the market in 2024. Key Growth Drivers Rising R&D costs and long timelines of traditional clinical trials. Increasing adoption of AI, machine learning, and computational biology in pharma. Regulatory encouragement of model-informed drug development approaches. Growing need to reduce animal testing and ethical concerns. Expansion of digital health platforms enabling data integration. Market Segmentation Analysis By Technology: Computational modeling and simulation software lead the market. AI/ML-driven analytics and predictive modeling are rapidly expanding. By Application: Drug discovery and efficacy testing dominate, with growing use in toxicology and dosage optimization. By End User: Pharmaceutical companies and Contract Research Organizations (CROs) are primary users. Regulatory bodies and academic institutions contribute to development and validation. Request for Customized Sample Report as per Your Business Requirement: https://www.datamintelligence.com/customize/in-silico-clinical-trials-market Latest FDA Approvals and Drug Approvals 1. FDA has broadened guidance accepting computational modeling and verified virtual evidence to support device 510(k) submissions and biologic IND filings, reflecting regulatory endorsement of in-silico data. 2. Insilico Medicine received FDA IND clearance for ISM5939, an AI-designed oral small molecule targeting solid tumors, marking a landmark drug approval leveraging in-silico design. 3. FDA accepted in-silico PK/PD simulation data from Pfizer for tofacitinib formulations, reducing need for redundant phase 3 clinical trials report Regional Insights North America: Largest market valued at USD 220 million in 2024, driven by strong pharma presence and technological leadership. Europe: Increasing investments in computational biology and clinical trial modernization initiatives. Asia-Pacific: Emerging market with growing clinical research outsourcing and digital transformation. Key Players Key companies include Certara || Dassault Systèmes || InSilicoTrials Technologies || Nova || Insilico Medicine || The AnyLogic Company || Simulations Plus || VeriSIM Life || Physiomics Plc, and ANSYS, Inc. Firms focus on enhancing simulation accuracy, expanding platform capabilities, and regulatory engagement. Recent Developments In May 2025, the FDA outlined a major transition away from animal testing, citing that more than 90% of drugs that succeed in animal studies fail in human trials. The updated roadmap prioritizes new approach methodologies, including human-based in vitro systems, in-silico modeling, and advanced platforms for evaluating immunogenicity, toxicity, and pharmacodynamic responses. Certara's new in-silico trial platform launched for oncology drug modeling (Q2 2025). Partnerships between pharma and software developers to integrate real-world data. Regulatory agencies issuing new guidelines supporting in-silico evidence submission. Buy This Report with Year-End Offer (Buy 1 report: Get 30% OFF | Buy 2 reports: Get 50% OFF each! Limited time offer): https://www.datamintelligence.com/buy-now-page?report=in-silico-clinical-trials-market Recent Procurements and Market Movement 1. Tempus AI acquired Deep 6 AI in March 2025, integrating real-time EMR analytics with AI-driven virtual patient matching to accelerate clinical trial recruitment. 2. CROs expanded in-silico trial services, with Worldwide Clinical Trials partnering with Medidata to combine eSource capture and virtual-patient simulation, increasing trial speed and reducing cost. 3. Investments in AI-driven drug discovery platforms including Absci, Owkin, and Recursion raised hundreds of millions, boosting computational modeling for preclinical and clinical workflows. Market Outlook In-silico clinical trials will significantly disrupt traditional drug development by lowering costs, improving prediction accuracy, and enabling personalized medicine, with adoption accelerating worldwide through 2032. Related Reports Clinical Trials Market Reaches $143B by 2033 - DataM Intelligence @ https://www.datamintelligence.com/research-report/clinical-trials-market AI in Clinical Trials Market Growing to $5B by 2033 @ https://www.datamintelligence.com/research-report/ai-in-clinical-trials-market Sai Kiran DataM Intelligence 4market Research LLP sai.k@datamintelligence.com Visit us on social media: LinkedIn X Legal Disclaimer: EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Insilico Medicine Frequently Asked Questions (FAQ)
When was Insilico Medicine founded?
Insilico Medicine was founded in 2014.
Where is Insilico Medicine's headquarters?
Insilico Medicine's headquarters is located at Phase 2, Hong Kong Science Park, Pak Shek Kok, New Territories.
What is Insilico Medicine's latest funding round?
Insilico Medicine's latest funding round is Series E.
How much did Insilico Medicine raise?
Insilico Medicine raised a total of $524.8M.
Who are the investors of Insilico Medicine?
Investors of Insilico Medicine include Warburg Pincus, OrbiMed, Prosperity7 Ventures, Puxing Synergy Fund, Pudong VC and 41 more.
Who are Insilico Medicine's competitors?
Competitors of Insilico Medicine include METiS Technologies, AIBODY, InSilicoTrials, Aqemia, Variational AI and 7 more.
What products does Insilico Medicine offer?
Insilico Medicine's products include Pharma.AI and 1 more.
Who are Insilico Medicine's customers?
Customers of Insilico Medicine include Exelixis, Stemline Therapeutics , Sanofi and Tenacia.
Loading...
Compare Insilico Medicine to Competitors

Atomwise develops machine learning-based discovery engines and uses artificial intelligence (AI)-based neural networks to help discover new medicines. It predicts drug candidates for pharmaceutical companies, start-ups, and research institutions, and designs drugs using computational drug design. It was formerly known as Chematria. The company was founded in 2012 and is based in San Francisco, California.

Insitro is a data-driven drug discovery and development company that utilizes machine learning and high-throughput biology. The company has a machine learning platform that combines in vitro cellular data with human clinical data to identify insights and interventions in metabolism, oncology, and neuroscience. Insitro's approach uses technology to model disease states and design therapeutic interventions. It was founded in 2018 and is based in South San Francisco, California.

Aqemia focuses on transforming the drug discovery process. The company offers technology that combines quantum-inspired physics and machine learning to scale the drug discovery process, generating a rapidly growing pipeline of new drug candidates. It primarily sells to the pharmaceutical industry. It was founded in 2019 and is based in Paris, France.
SOM Biotech operates as a clinical-stage drug discovery and development company that focuses on the treatment of rare Central Nervous System disorders. The company utilizes an artificial intelligence platform to discover and develop drugs, with emphasis on orphan diseases and repurposing existing drugs for new therapeutic indications. SOM Biotech's lead product has completed Phase 2b trials for Huntington's Disease. It was founded in 2009 and is based in Barcelona, Spain.

ProclaimRx operates in the biopharmaceutical sector and provides AI-driven solutions for sales and marketing teams. It focuses on workflows that support healthcare professional interactions. ProclaimRx serves the biopharmaceutical industry and uses its knowledge in health tech platforms and AI to offer decision support and sales force solutions. It was founded in 2023 and is based in New York, New York.

Cradle focuses on protein engineering in the biotechnology sector, using machine learning to optimize protein sequences. It provides tools for designing improved variants of target proteins, integrating laboratory data, and predicting performance to support research and development. Its solutions are relevant for biotech teams working on various applications, including therapeutics, enzymes, vaccines, peptides, and antibodies. It was founded in 2021 and is based in Amsterdam, Netherlands.
Loading...