
IntraBio
Founded Year
2015Stage
Unattributed - II | AliveTotal Raised
$50.57MValuation
$0000Last Raised
$40.27M | 2 yrs agoAbout IntraBio
IntraBio is involved in the discovery and development of drugs for rare neurodegenerative diseases within the pharmaceutical industry. The company is working on therapies that aim to address medical needs and patient outcomes. Its lead product, IB1001, is an orally administered modified amino acid intended for the treatment of these conditions. It was founded in 2015 and is based in Austin, Texas.
Loading...
Loading...
Expert Collections containing IntraBio
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
IntraBio is included in 1 Expert Collection, including Unicorns- Billion Dollar Startups.
Unicorns- Billion Dollar Startups
1,309 items
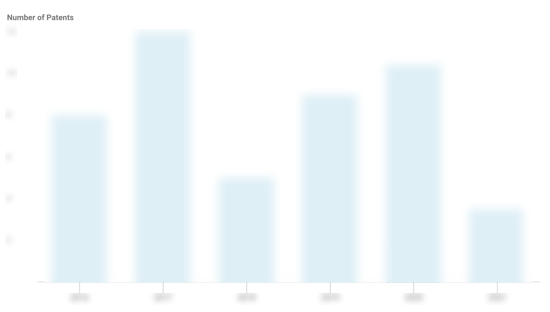
IntraBio Patents
IntraBio has filed 22 patents.
The 3 most popular patent topics include:
- rare diseases
- autosomal recessive disorders
- neurological disorders
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
8/11/2017 | 11/19/2024 | Rare diseases, Autosomal recessive disorders, Neurological disorders, Neurodegenerative disorders, Syndromes | Grant |
Application Date | 8/11/2017 |
|---|---|
Grant Date | 11/19/2024 |
Title | |
Related Topics | Rare diseases, Autosomal recessive disorders, Neurological disorders, Neurodegenerative disorders, Syndromes |
Status | Grant |
Latest IntraBio News
Oct 17, 2025
was valued at USD 260 million in 2024 and is predicted to hit around USD 456.42 million by 2034, rising at a 5.75% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research. Numerous factors influence market growth, including the rising incidence of rare disorders, growing research and development activities, and the need for personalized medicines. Scientists adopt advanced technologies for the effective treatment of the effective treatment of Sandhoff disease. Government organizations launch initiatives to encourage people to seek early diagnosis, enabling healthcare professionals to provide early intervention. The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/5843 The Sandhoff Disease Treatment Market: Highlights North America dominated the global market share by 48% in 2024. Asia-Pacific is expected to grow at the fastest CAGR in the market during the forecast period. By therapy type, the supportive & symptomatic treatment segment held the largest revenue share of the market in 2024. By therapy type, the gene therapy segment is expected to expand rapidly in the market in the coming years. By disease form type, the infantile Sandhoff disease segment accounted for the highest revenue share of the market in 2024. By disease form type, the adult Sandhoff disease segment is expected to show the fastest growth over the forecast period. By drug type, the anticonvulsants & muscle relaxants segment led the Sandhoff disease treatment market in 2024. By drug type, the experimental gene therapies segment is expected to grow with the highest CAGR in the market during the studied years. By route of administration, the oral segment held a major revenue share of the market in 2024. By route of administration, the intrathecal/intravenous segment is expected to grow at the fastest CAGR in the market during the forecast period. By end-user, the hospitals & specialized clinics segment contributed the biggest revenue share of the market in 2024. By end-user, the academic medical centers segment is expected to witness the fastest growth in the market over the forecast period. What is Sandhoff Disease Treatment? The Sandhoff disease treatment market refers to the development, manufacturing, and commercialization of treatment regimens for Sandhoff disease. Sandhoff disease is a rare genetic disorder in infants that is caused due to a lack of beta-hexosaminidase. It is a lysosomal storage disorder that destroys nerve cells in the brain and spinal cord. Common treatment modalities include anticonvulsants, bone marrow transplants, cell and gene therapy, and substrate reduction therapy. Market Scope Metric Details Market Size in 2025USD 274.95 Million Projected Market Size in 2034USD 456.42 Million CAGR (2025 - 2034)5.75 % Leading Region North America Share by 48% Market SegmentationBy Therapy Type, By Disease Form, By Drug Type, By End User, By Region Top Key Players Denali Therapeutics, Sio Gene Therapies (formerly Axovant Gene Therapies), Taysha Gene Therapies, Passage Bio, Orchard Therapeutics, REGENXBIO Inc., uniQure N.V., Sanofi S.A., Johnson & Johnson (Janssen R&D), Takeda Pharmaceutical Company, Novartis AG, Amicus Therapeutics, Biomarin Pharmaceutical Inc., Pfizer Inc. (gene therapy platforms), Genzyme (Sanofi division), Bluebird Bio, Cure Rare Disease (CRD), M6P Therapeutics, IntraBio Inc., National Institutes of Health (NIH) You can place an order or ask any questions, please feel free to contact us at Booming Clinical Trials: Major Potential Clinical trials are conducted to assess the safety and efficacy of novel treatment regimens for Sandhoff disease. The increasing clinical trials is a result of growing research and development activities. As of October 2025, a total of 25 clinical trials were registered on the clinicaltrials.gov website related to Sandhoff disease. Current clinical trials focus on evaluating gene therapy candidates and repurposing licensed medications for Sandhoff disease. Researchers also evaluate the pharmacokinetics and pharmacodynamic properties of novel drugs. Lack of Effective Treatments: Major Limitation Currently, there are no specific cures for Sandhoff disease. The drugs prescribed by healthcare professionals can only provide symptomatic relief. Effective treatment regimens are not available that can enter the brain. This leads to the buildup of fatty substances in the brain, causing neurological damage. The Sandhoff Disease Treatment Market: Regional Analysis North America held a major revenue share by 48% of the market in 2024. The presence of key players, the availability of state-of-the-art research and development facilities, and favorable government support are the major growth factors of the market in North America. Government organizations support the development of personalized medicines for rare disorders through initiatives and funding. The presence of an advanced clinical trial infrastructure encourages researchers to develop innovative therapeutics. The U.S. states conduct newborn screening programs to screen newborns for many serious disorders, including spinal muscular atrophy, cystic fibrosis, lysosomal storage diseases, and immunodeficiencies. The estimated prevalence of Sandhoff disease in the U.S. is between 1 in 500,000 and 1 in 1,500,000. Canada’s Drug Agency recently published a new guidance to support newborn screening programs in Canada to support the National Strategy for Drugs for Rare Diseases. In November 2024, McMaster researchers identified the role of 4-phenylbutyric acid (4-PBA), an FDA-approved product for another condition, for the treatment of Sandhoff and Tay-Sachs diseases. Download the single region market report @ https://www.towardshealthcare.com/checkout/5843 Asia-Pacific is expected to witness the fastest growth in the Sandhoff disease treatment market during the predicted timeframe. The increasing incidence of rare disorders and the growing demand for personalized medicines augment the market. The burgeoning biopharmaceutical sector and the increasing investment also contribute to market growth. Countries like China, India, and Japan are at the forefront of developing treatment modalities for rare diseases through government support. Additionally, the rising adoption of advanced technologies and the increasing collaboration among key players foster market growth. The Indian government’s National Fund for Rare Diseases (NFRD) has a budget of Rs 974 crore for 2024-25 and 2025-26 for a centralized information portal and enhanced public awareness. The Ministry of Health and Family Welfare (MoHFW) announced a similar or higher amount for 2026-27 and 2027-28. The Sandhoff Disease Treatment Market: Segmentation Analysis By Therapy Type The supportive & symptomatic treatment segment held a dominant presence in the market in 2024, due to a lack of effective treatment regimens. Supportive care in acute infantile Sandhoff disease focuses on providing adequate nutrition and hydration. It is comparatively cost-effective, enhancing patient convenience. The gene therapy segment is expected to grow at the fastest CAGR in the market during the forecast period. Gene therapy offers unprecedented advantages to treat any kind of rare disorder, including Sandhoff disease. Out of the total 25 clinical trials for Sandhoff disease, 3 are based on evaluating gene therapy for its treatment. Become a valued research partner with us - https://www.towardshealthcare.com/schedule-meeting By Disease Form Type The infantile Sandhoff disease segment held the largest revenue share of the Sandhoff disease treatment market in 2024, due to hereditary reasons, i.e., both parents carry the gene causing Sandhoff disease that is passed onto the child. Infantile Sandhoff disease is the most common type, affecting children less than 6 months of age. It results in slow developmental progress and severe neurologic impairment. The adult Sandhoff disease segment is expected to grow with the highest CAGR in the market during the studied years. Late-onset Sandhoff disease is caused in older teens and young adults, leading to certain neurologic and psychiatric findings. Psychiatric medications, such as haloperidol and chlorpromazine, are estimated to worsen the condition in adults. The growing awareness of disease diagnosis boosts the segment’s growth. By Drug Type The anticonvulsants & muscle relaxants segment contributed the biggest revenue share of the Sandhoff disease treatment market in 2024, due to the need to manage seizures and spasticity. As Sandhoff disease affects nerve functioning, anticonvulsants and muscle relaxants prove to be beneficial in providing symptomatic relief to patients, improving the quality of life of individuals. The experimental gene therapies segment is expected to expand rapidly in the market in the coming years. Gene therapy is developed to introduce a functional copy of the HEXB gene to restore enzyme activity. Gene therapies for Sandhoff disease are currently under investigation, and more research is needed for human trials. By Route of Administration The oral segment accounted for the highest revenue share of the Sandhoff disease treatment market in 2024, due to enhanced patient convenience and cost-effective treatments. The oral route eliminates the need for skilled professionals to administer therapeutics. Anticonvulsants and antispasmodics are usually administered through the oral route. They result in fewer side effects compared to the intravenous route. The intrathecal/intravenous segment is expected to witness the fastest growth in the market over the forecast period. The intravenous route is essential to deliver innovative gene therapies as they are unstable through the oral route. It results in higher bioavailability and prevents the first-pass metabolism. Drugs delivered through this route lead to a faster onset of action. Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership By End-User The hospitals & specialized clinics segment led the Sandhoff disease treatment market in 2024, due to the presence of favorable infrastructure and suitable capital investments. Hospitals and specialized clinics possess skilled professionals to provide personalized treatment and care to patients. They also have specialized equipment for the diagnosis, treatment, and rehabilitation of Sandhoff disease. They conduct clinical trials, benefiting patients by accessing novel therapeutics before market approval. The academic medical centers segment is expected to show the fastest growth over the forecast period. Academic medical centers conduct research activities and clinical trials for patients with Sandhoff disease. They provide affordable treatment and have trained professionals. Top Companies & Their Contributions in the Market
IntraBio Frequently Asked Questions (FAQ)
When was IntraBio founded?
IntraBio was founded in 2015.
Where is IntraBio's headquarters?
IntraBio's headquarters is located at 201 West 5th Street, Austin.
What is IntraBio's latest funding round?
IntraBio's latest funding round is Unattributed - II.
How much did IntraBio raise?
IntraBio raised a total of $50.57M.
Loading...
Loading...
