Lindus Health
Founded Year
2021Stage
Grant - II | AliveTotal Raised
$79.7MMosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
+83 points in the past 30 days
About Lindus Health
Lindus Health focuses on clinical trials for the life sciences sector, providing services that include trial design, patient recruitment, data management, and study monitoring. The company operates with fixed-price quotes and milestone-based payments. Lindus Health serves biotech and pharmaceutical companies, using its software platform and access to a database of electronic health records. It was founded in 2021 and is based in New York, New York.
Loading...
Lindus Health's Product Videos


ESPs containing Lindus Health
The ESP matrix leverages data and analyst insight to identify and rank leading companies in a given technology landscape.
The clinical trial management systems market provides a range of software solutions that enable the management of clinical trials, including in protocol design, study planning, participant recruitment, data collection and analysis, and regulatory compliance. These systems can help streamline the clinical trial process, reduce administrative burden, and improve data quality and accuracy. Many of th…
Lindus Health named as Challenger among 15 other companies, including Oracle, Medidata, and Veeva Systems.
Loading...
Research containing Lindus Health
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Lindus Health in 3 CB Insights research briefs, most recently on Oct 3, 2025.

Oct 3, 2025 report
Book of Scouting Reports: AI in Clinical Development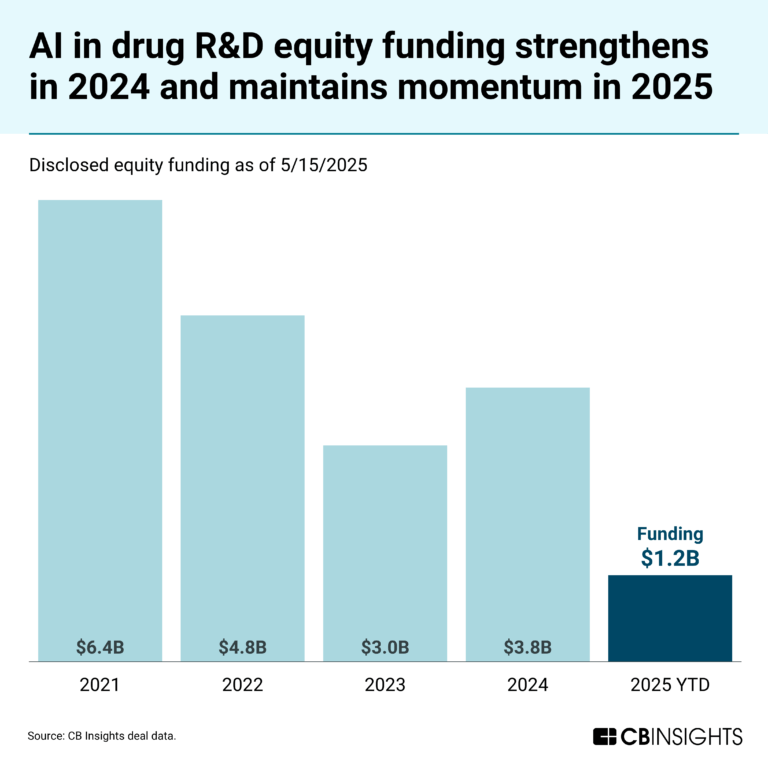
May 23, 2025
The AI in drug R&D market mapExpert Collections containing Lindus Health
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Lindus Health is included in 1 Expert Collection, including Digital Health.
Digital Health
12,122 items
The digital health collection includes vendors developing software, platforms, sensor & robotic hardware, health data infrastructure, and tech-enabled services in healthcare. The list excludes pureplay pharma/biopharma, sequencing instruments, gene editing, and assistive tech.
Latest Lindus Health News
Oct 27, 2025
By News Desk Published On: October 27, 2025Last Updated: October 27, 2025 Join industry leaders Meri Beckwith, Malcolm Fogarty from Lindus Health and Dr Thomas Gurry from Myota for a live webinar on how consumer health brands can run faster, leaner and claims-ready clinical trials. Secure your spot today . In consumer health, clinical evidence is now the currency of competitive advantage. Consumers are better informed and more discerning than ever. They expect proof that products deliver real results, and they’re quick to turn away from brands that can’t back up their claims. In this environment, credible, transparent clinical evidence is what differentiates leaders. Yet many brands still face a common challenge: traditional, pharma-style trials are too slow, too costly, and poorly suited to the realities of consumer health. Rigid protocols and lengthy timelines often miss what really matters—real-world consumer outcomes. A new model for generating science-backed evidence Innovative sponsors are now building trials explicitly designed for consumer health: faster, leaner and engineered for richer insights. These modern approaches balance speed, cost, and scientific credibility, delivering data that meets regulatory standards and drives commercial success. Today’s most successful brands are breaking from that model, embracing right-sized, claims-first trials. This modern approach centers on three key principles that make consumer health research faster, smarter and commercially impactful. 1. Claims-first design Effective consumer health studies begin with the claims that will ultimately appear on packaging, marketing, or retail. Trial protocols are increasingly aligned with those intended claims, linking each to measurable, regulator-accepted endpoints. 2. Lean, hybrid execution. Digital tools like eConsent, remote monitoring and targeted online recruitment are transforming timelines. Decentralized and adaptive designs enable more inclusive studies that reflect real consumer use, without compromising data quality. 3. Real-world scalability Evidence strategies are also being designed with global reach in mind. Harmonised protocols and adaptive designs allow sponsors to collect and analyse data consistently across markets, ensuring claims remain credible and translatable from one region to another. Learn from consumer health industry leaders In this upcoming webinar , Meri Beckwith and Malcolm Fogarty from Lindus Health, together with Dr Thomas Gurry from Myota, will share how innovative trial designs, from decentralized studies to real-world evidence approaches, are transforming clinical validation for consumer health brands. You’ll gain practical insights on how to: Generate medical-grade data at consumer-scale budgets Balance speed, cost, and credibility in trial design Turn credible clinical evidence into commercial advantage Register here to learn how leading consumer health brands are using science-backed trials to strengthen claims and build lasting consumer trust.
Lindus Health Frequently Asked Questions (FAQ)
When was Lindus Health founded?
Lindus Health was founded in 2021.
Where is Lindus Health's headquarters?
Lindus Health's headquarters is located at 228 East 45th Street, New York.
What is Lindus Health's latest funding round?
Lindus Health's latest funding round is Grant - II.
How much did Lindus Health raise?
Lindus Health raised a total of $79.7M.
Who are the investors of Lindus Health?
Investors of Lindus Health include The Advanced Research and Invention Agency, Seedcamp, Firstminute Capital, Creandum, Visionaries Club and 24 more.
Who are Lindus Health's competitors?
Competitors of Lindus Health include Biorce, Current Health, Castor, Huma, Aparito and 7 more.
Loading...
Compare Lindus Health to Competitors

Medable specializes in the development of decentralized clinical trial software and electronic Clinical Outcome Assessment (eCOA) solutions within the healthcare technology sector. The company's platform provides tools for remote monitoring, data collection, and trial management, utilizing artificial intelligence (AI) to improve processes in clinical research. Medable serves the pharmaceutical and biotechnology industries, including mid to large pharma, emerging biotech firms, and contract research organizations (CROs). Medable was formerly known as Dermatrap. It was founded in 2012 and is based in Palo Alto, California.

ObvioHealth provides digital health solutions within the clinical trial industry. The company has a platform and mobile application allows for remote monitoring and participation in clinical trials, focusing on data collection and participant engagement. ObvioHealth's services are relevant to the healthcare sector, especially in trial management. It was founded in 2017 and is based in New York, New York.

Huma is a healthcare AI company that provides digital health solutions for care and research. The company offers services including remote patient monitoring, hospital-at-home programs, virtual wards, and decentralized clinical trials. Huma's technology is used by hospitals and clinics globally and is integrated into clinical trials. Huma was formerly known as Huma Therapeutics GmbH. It was founded in 2011 and is based in London, United Kingdom.

THREAD is a company focused on clinical research and electronic clinical outcome assessments (eCOA) within the life sciences sector. It offers a proprietary decentralized research platform and a suite of supporting services designed to enable remote data capture from participants and sites during clinical studies. It was founded in 2005 and is based in Tustin, California.

Evinova focuses on digital health solutions within the life sciences sector. The company provides products intended to support the clinical development lifecycle, including the unified trial solution and drug development suite, which are used for remote patient monitoring, electronic clinical outcome assessments, and data management in clinical trials. Evinova serves the pharmaceutical, biotech, and contract research organization industries with its digital solutions. It was founded in 2023 and is based in Baar, Switzerland.

Reify Health provides digital solutions and infrastructure within the healthcare and pharmaceutical sectors to support the clinical trials ecosystem. The company offers cloud-based software aimed at trial enrollment processes and supplies the infrastructure required for clinical research. Reify Health serves biopharma companies, research clinics, and healthcare and community organizations. Reify Health was formerly known as ZeroSum Health. It was founded in 2012 and is based in Boston, Massachusetts.
Loading...