
Medable
Founded Year
2012Stage
Series D | AliveTotal Raised
$533.75MValuation
$0000Last Raised
$304M | 4 yrs agoMosaic Score The Mosaic Score is an algorithm that measures the overall financial health and market potential of private companies.
-47 points in the past 30 days
About Medable
Medable specializes in the development of decentralized clinical trial software and electronic Clinical Outcome Assessment (eCOA) solutions within the healthcare technology sector. The company's platform provides tools for remote monitoring, data collection, and trial management, utilizing artificial intelligence (AI) to improve processes in clinical research. Medable serves the pharmaceutical and biotechnology industries, including mid to large pharma, emerging biotech firms, and contract research organizations (CROs). Medable was formerly known as Dermatrap. It was founded in 2012 and is based in Palo Alto, California.
Loading...
ESPs containing Medable
The ESP matrix leverages data and analyst insight to identify and rank leading companies in a given technology landscape.
The decentralized clinical trials platforms market specifically encompasses software and technology solutions designed to facilitate and manage remote clinical trials. These platforms integrate various components such as telemedicine, mobile health applications, and wearable device data collection to enable trial activities without frequent site visits. By streamlining remote participation, these …
Medable named as Highflier among 15 other companies, including Veeva Systems, IQVIA, and Amazon Web Services.
Medable's Products & Differentiators
Medable Platform
To create a seamless experience for site and patients, all of our products are integrated into a single platform. The core Platform contains portals for the site, patient, and sponsor in both web and mobile modalities.
Loading...
Research containing Medable
Get data-driven expert analysis from the CB Insights Intelligence Unit.
CB Insights Intelligence Analysts have mentioned Medable in 4 CB Insights research briefs, most recently on Oct 3, 2025.

Oct 3, 2025 report
Book of Scouting Reports: AI in Clinical Development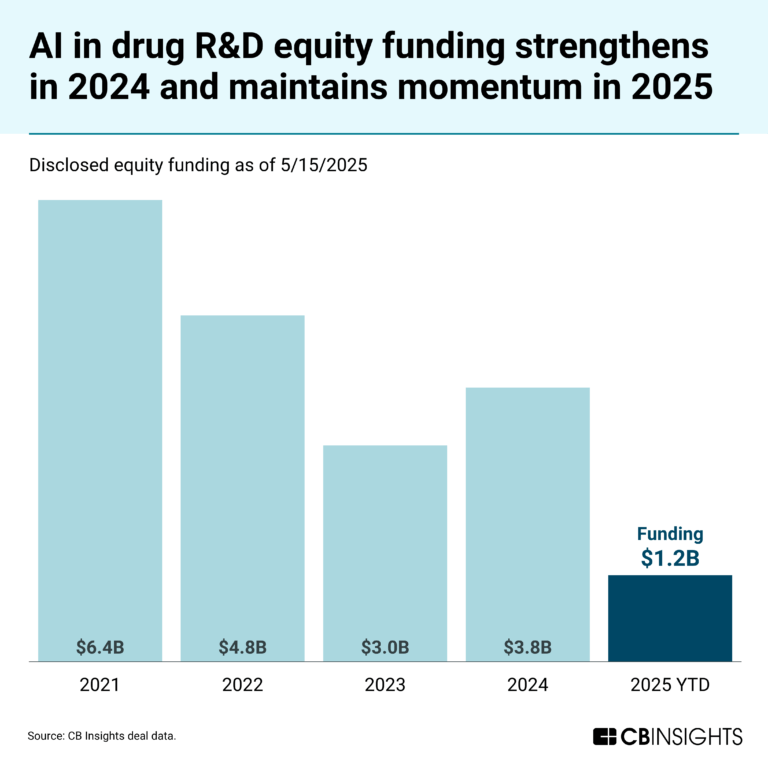
May 23, 2025
The AI in drug R&D market map
Aug 21, 2024
The clinical trials tech market map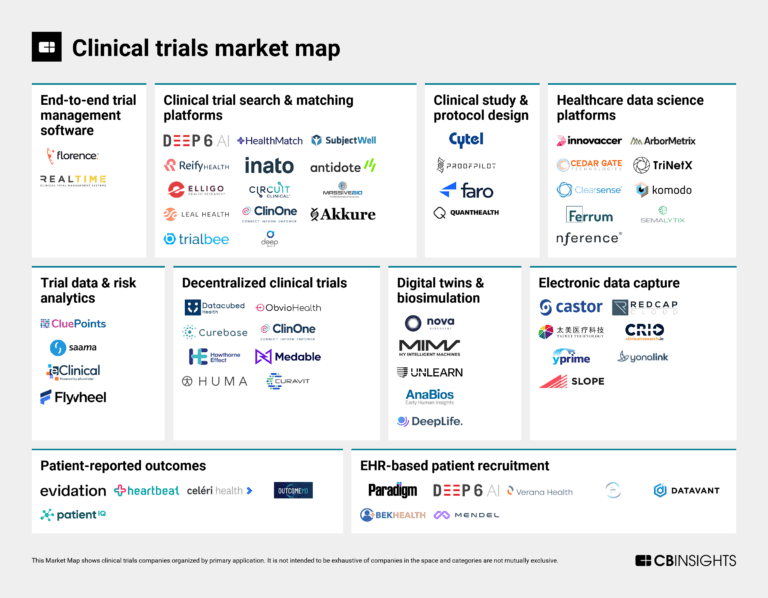
Aug 1, 2023
The clinical trials market mapExpert Collections containing Medable
Expert Collections are analyst-curated lists that highlight the companies you need to know in the most important technology spaces.
Medable is included in 7 Expert Collections, including Unicorns- Billion Dollar Startups.
Unicorns- Billion Dollar Startups
1,309 items
Regtech
1,453 items
Technology that addresses regulatory challenges and facilitates the delivery of compliance requirements. Regulatory technology helps companies and regulators address challenges ranging from compliance (e.g. AML/KYC) automation and improved risk management.
Conference Exhibitors
5,302 items
Digital Health 50
300 items
The winners of the second annual CB Insights Digital Health 150.
Digital Health
12,122 items
The digital health collection includes vendors developing software, platforms, sensor & robotic hardware, health data infrastructure, and tech-enabled services in healthcare. The list excludes pureplay pharma/biopharma, sequencing instruments, gene editing, and assistive tech.
Telehealth
3,123 items
Companies developing, offering, or using electronic and telecommunication technologies to facilitate the delivery of health & wellness services from a distance. *Columns updated as regularly as possible; priority given to companies with the most and/or most recent funding.
Medable Patents
Medable has filed 5 patents.
The 3 most popular patent topics include:
- health informatics
- healthcare occupations
- medical terminology
Application Date | Grant Date | Title | Related Topics | Status |
|---|---|---|---|---|
6/21/2022 | 2/13/2024 | Computer security, Information privacy, Data security, Computer network security, Access control | Grant |
Application Date | 6/21/2022 |
|---|---|
Grant Date | 2/13/2024 |
Title | |
Related Topics | Computer security, Information privacy, Data security, Computer network security, Access control |
Status | Grant |
Latest Medable News
Oct 15, 2025
Expected to grow to $4.18 billion in 2029 at a compound annual growth rate (CAGR) of 28.4%” — The Business Research Company LONDON, GREATER LONDON, UNITED KINGDOM, October 15, 2025 / EINPresswire.com / -- "Get 20% Off All Global Market Reports With Code ONLINE20 – Stay Ahead Of Trade Shifts, Macroeconomic Trends, And Industry Disruptors What Is The Projected Market Size & Growth Rate Of The Virtual Clinical-Trial Participant Avatars Market? The market for virtual participant avatars in clinical trials has seen significant growth in the past few years. This industry is projected to expand from $1.19 billion in 2024 to $1.54 billion in 2025, boasting a compound annual growth rate (CAGR) of 28.8%. Factors leading to such growth during the historic period include the increasing embrace of decentralized clinical trials, growing requirement for diverse patient representation in trials, expansion of wearable health monitoring device usage, regulatory backing for digital trial solutions, and the urgency to shorten clinical trial durations. The market size for avatars used in virtual clinical trials is predicted to witness a massive surge in growth in the upcoming years, reaching a value of $4.18 billion by 2029, with a compound annual growth rate (CAGR) of 28.4%. This significant growth during the forecast period can be credited to the progress in AI-driven patient simulation, the convergence of virtual avatars with electronic data capture systems, the growing emphasis on personalized medicine, increased investments in digital twin technologies, and the broadening of virtual trials for rare diseases and chronic ailments. Key trends anticipated during this period include applying AI-powered digital twins for patient simulation, the incorporation of real-life data and electronic health records, the use of machine learning to predict trial outcomes, the creation of immersive VR/AR platforms for enhancing patient engagement, and the employment of blockchain for the secure management of patient data. Download a free sample of the virtual clinical-trial participant avatars market report: https://www.thebusinessresearchcompany.com/sample.aspx?id=28351&type=smp What Is The Crucial Factor Driving The Global Virtual Clinical-Trial Participant Avatars Market? The expansion of the virtual clinical-trial participant avatars market is anticipated to be driven by the increasing number of clinical trials. Clinical trials, which are structured studies designed to assess the safety and effectiveness of medical interventions in human subjects, are on the rise due to amplified pharmaceutical investment in pharmaceutical development. This is due to the quest for novel treatments for complicated illnesses and personalized medicine methods. Avatars of clinical-trial participants generate AI-enabled digital patient profiles that mimic disease progression, treatment reaction, and safety results. These assist in quicker recruitment, intelligent trial design, earlier detection of effective therapies, and minimized expenses and risks. For instance, as reported by the Association of the British Pharmaceutical Industry, a trade organization based in the UK in November 2023, the total number of industry-funded clinical trials initiated in the UK annually rose modestly by 4.3%, escalating from 394 trials in 2021 to 411 trials in 2022. Hence, the escalation in the number of clinical trials is spurring the growth of the virtual clinical-trial participant avatars market. Who Are The Emerging Players In The Virtual Clinical-Trial Participant Avatars Market? Major players in the Virtual Clinical-Trial Participant Avatars Global Market Report 2025 include: • NVIDIA Corporation • Dassault Systèmes SE • Signant Health Holding Corp. • Owkin Inc • Medable Inc. • Insilico Medicine Inc. • Science 37 Inc. • THREAD Inc. • Lindus Health Limited • Obvio Health What Are The Main Trends, Positively Impacting The Growth Of Virtual Clinical-Trial Participant Avatars Market? Major corporations in the virtual clinical trial participant avatars sector are turning their attention towards the development of cutting-edge solutions, such as digital twin-based clinical trial participants. These innovative solutions aim to boost the effectiveness of trials and enhance the precision in predicting drug development outcomes. By simulating real patients, digital twin-based clinical trial participants replicate their physiological and behavioral reactions, allowing researchers to predict the results of treatments and fine-tune trial designs, thereby decreasing the need for extensive physical testing. An illustration of this innovation is the update launched by the US-based AI clinical trial technology firm, Unlearn.ai, Inc., in July 2023. They unveiled an updated digital twin generator (DTG) for Alzheimer's disease, 3.1 version, which significantly improves the handling of missing baseline data by incorporating a neural network imputation model within a Neural Boltzmann Machine structure. This led to less sensitivity towards missing data and an overall superior performance. The DTG draws upon various patient data sets to generate individualized digital twins that can accurately forecast clinical and biomarker trajectories, thereby aiding clinical trials with a more exact and robust modeling of Alzheimer's progression. What Segments Are Covered In The Virtual Clinical-Trial Participant Avatars Market Report? The virtual clinical-trial participant avatars market covered in this report is segmented as 1) By Avatar Type: Artificial Intelligence-Generated Avatars, Digitally Modeled Human Avatars, Hybrid Avatars 2) By Deployment Mode: Cloud-Based, On-Premises 3) By Application: Drug Development, Patient Recruitment, Data Simulation, Remote Monitoring, Other Applications 4) By End-User: Pharmaceutical Companies, Contract Research Organizations, Academic And Research Institutes, Other End-Users Subsegments: 1) By Artificial Intelligence-Generated Avatars: Machine Learning-Based Avatars, Deep Learning-Based Avatars, Natural Language Processing (NLP)-Enabled Avatars, Generative Adversarial Network (GAN)-Based Avatars 2) By Digitally Modeled Human Avatars: Three-Dimensional (3D) Scanned Human Avatars, Motion Capture Modeled Avatars, Photorealistic Rendered Avatars, Anatomically Accurate Human Avatars 3) By Hybrid Avatars: artificial intelligence (AI)-Enhanced Digitally Modeled Avatars, Mixed Reality (MR) Enabled Avatars, Cognitive Simulation Avatars, Sensor-Integrated Virtual Avatars View the full virtual clinical-trial participant avatars market report: https://www.thebusinessresearchcompany.com/report/virtual-clinical-trial-participant-avatars-global-market-report Which Region Is Projected To Hold The Largest Market Share In The Global Virtual Clinical-Trial Participant Avatars Market? In the year 2024, the Virtual Clinical-Trial Participant Avatars Global Market Report indicated North America as the dominant region. The projected growth outlook suggests that Asia-Pacific will experience the quickest expansion during the forecast period. The report incorporates a thorough examination of regions including Asia-Pacific, Western Europe, Eastern Europe, North America, South America, the Middle East, and Africa. Browse Through More Reports Similar to the Global Virtual Clinical-Trial Participant Avatars Market 2025, By The Business Research Company Virtual Clinical Trials Global Market Report 2025 https://www.thebusinessresearchcompany.com/report/virtual-clinical-trials-global-market-report Patient Simulators Global Market Report 2025 https://www.thebusinessresearchcompany.com/report/patient-simulators-global-market-report Virtual Human Anatomy Software Global Market Report 2025 https://www.thebusinessresearchcompany.com/report/virtual-human-anatomy-software-global-market-report Speak With Our Expert: Saumya Sahay Americas +1 310-496-7795 Asia +44 7882 955267 & +91 8897263534 Europe +44 7882 955267 Email: saumyas@tbrc.info The Business Research Company - www.thebusinessresearchcompany.com Follow Us On: • LinkedIn: https://in.linkedin.com/company/the-business-research-company Oliver Guirdham The Business Research Company info@tbrc.info Visit us on social media: LinkedIn Facebook X Legal Disclaimer: EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Medable Frequently Asked Questions (FAQ)
When was Medable founded?
Medable was founded in 2012.
Where is Medable's headquarters?
Medable's headquarters is located at 525 University Avenue, Palo Alto.
What is Medable's latest funding round?
Medable's latest funding round is Series D.
How much did Medable raise?
Medable raised a total of $533.75M.
Who are the investors of Medable?
Investors of Medable include Informed Ventures, Sapphire Ventures, Western Technology Investment, Tiger Global Management, Blackstone and 17 more.
Who are Medable's competitors?
Competitors of Medable include ObvioHealth, MediMergent, JNPMEDI, mpathic, Lindus Health and 7 more.
What products does Medable offer?
Medable's products include Medable Platform and 2 more.
Who are Medable's customers?
Customers of Medable include GSK and Syneos.
Loading...
Compare Medable to Competitors

Lindus Health focuses on clinical trials for the life sciences sector, providing services that include trial design, patient recruitment, data management, and study monitoring. The company operates with fixed-price quotes and milestone-based payments. Lindus Health serves biotech and pharmaceutical companies, using its software platform and access to a database of electronic health records. It was founded in 2021 and is based in New York, New York.

Reify Health provides digital solutions and infrastructure within the healthcare and pharmaceutical sectors to support the clinical trials ecosystem. The company offers cloud-based software aimed at trial enrollment processes and supplies the infrastructure required for clinical research. Reify Health serves biopharma companies, research clinics, and healthcare and community organizations. Reify Health was formerly known as ZeroSum Health. It was founded in 2012 and is based in Boston, Massachusetts.

Curebase is a company that provides an eClinical platform in the clinical research sector, offering tools such as electronic patient-reported outcomes (ePRO), electronic clinical outcome assessments (eCOA), electronic consent (eConsent), and electronic data capture (EDC) systems. The platform is designed for study launches, data collection, and participant engagement. It was founded in 2017 and is based in San Francisco, California.

ObvioHealth provides digital health solutions within the clinical trial industry. The company has a platform and mobile application allows for remote monitoring and participation in clinical trials, focusing on data collection and participant engagement. ObvioHealth's services are relevant to the healthcare sector, especially in trial management. It was founded in 2017 and is based in New York, New York.

THREAD is a company focused on clinical research and electronic clinical outcome assessments (eCOA) within the life sciences sector. It offers a proprietary decentralized research platform and a suite of supporting services designed to enable remote data capture from participants and sites during clinical studies. It was founded in 2005 and is based in Tustin, California.

Castor operates within the healthcare sector and provides a clinical trial platform designed for the design, deployment, patient engagement, data collection, and analysis of clinical trials. The platform is adaptable and can meet the requirements of different stakeholders in the clinical research process. It primarily serves the healthcare sector. The company was founded in 2012 and is based in Amsterdam, Netherlands.
Loading...
